The Challenge
This exciting start-up company were searching for a partner to help them to build a quick-turn prototype for First-in-Human (FIH) clinical trials using full sterilised devices. Due to the need to meet critical milestones for clinical procedures they needed the project completed inside 3 months, they were seeking a partner who could act quickly and efficiently in responding to their needs.
Once the prototype design was finalised, there would then be a need to quickly produce 300+ units for animal trials, followed by design verification, and finally into FIH clinical trials.
Our Solution
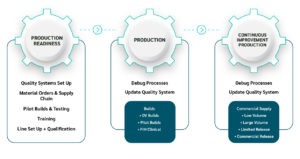
The ICS NPI team quickly implemented the device-building method into our quality systems, installed a production line, and trained our engineers to complete the prototypes and ship the devices to the customer for review.
After completion of prototyping builds, ICS used the learnings to debug and optimise the process. This approach ensures that all data and learnings are acted upon and become key in continuous improvement of the process. We implemented procedures / DMR / specifications and training via our own in-house quality systems, and these bespoke processes allow us to create and approve system documentation quickly.
From Concept to FIH
ICS Medical was flexible in ramping up the production line over two shifts and producing double output week-on-week, and the final part of the project was completed for sterilised devices for FIH clinical trials. The continual ramp-up by ICS Medical enabled the customer to meet all of their clinical milestones.
The customer was able to complete Design Verification within 8 weeks from production builds, and FIH was facilitated within a month thereafter.
The NPI department at ICS Medical successfully implemented the customer’s device to ICS’s procedures. Our engineers were able to react quickly to the challenge, providing flexibility on the tight timeline and ultimately ensuring that our customer’s needs and expectations were met and exceeded.
Following on from the success of achieving the customer requirements, we provided support for further pilot builds and next gen projects, and we continue to enjoy a collaborative and trusting relationship with the customer.
“We really enjoyed working with ICS from the start. We were under immense pressure with a strict deadline, but they successfully reached all our requirements. Their team was very professional, and we would be happy to work with them again. “
VP of R&D, Medical Device Start-up, North America.