At ICS Medical Devices we love a challenge – when a client brings us something that pushes the boundaries and tests the limits of what is currently possible.
Sometimes a client comes to us with an embryonic idea that requires nurturing, and sometimes they have a full device specification to take into prototyping. Wherever you are in the development lifecycle, our agile processes help accelerate your product efficiently towards your end goal – saving you time and money.
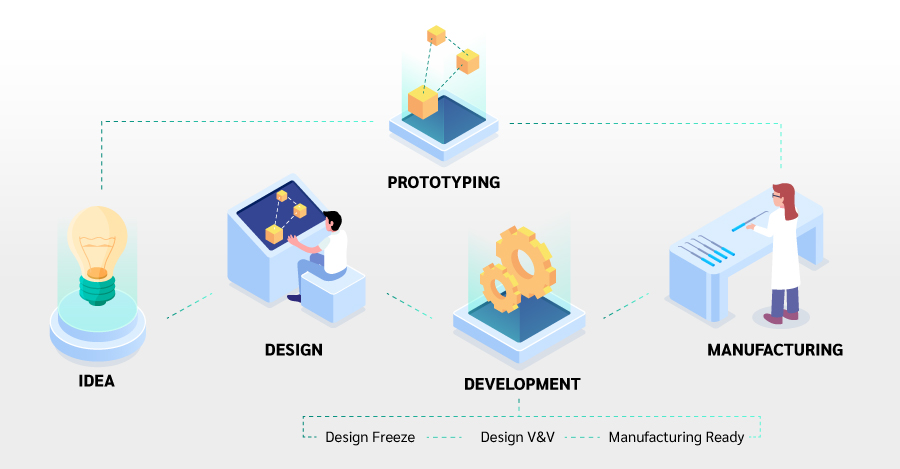
Below is an outline of the various stages of our approach. As stated above, depending on where you are in your journey you could drop into the process at any stage – Design, Prototyping, V&V, Manufacturing or otherwise.
Project Scoping
First, we need to define what the project is all about? We interrogate and question until we have a deep understanding of your needs and how we can apply our capabilities, so you obtain the best possible outcome.
From here we may go into Design and Development, or we may proceed straight to Prototyping.
Design
Design is where your idea is translated from a concept on paper to a proven and robust prototype and specification. The goal is to get design answers to the right questions and quickly facilitate informed decisions, thereby allowing us to iterate effectively and efficiently towards your final catheter design solution. Design stage is made up of 4 sub-steps:
- Plan/Specification
Define the scope, set the targets, and determine the deliverables for the project.
- Build/Prototype
Construct and build the concept prototype using our experience, expertise and capabilities. We will design and iterate on the spot if we see opportunity for improvement in the design, or if we see challenges and risks to your product.
As we set out at the beginning of this article, if you already have a specification we can skip past the brainstorming and design phase. We will review your specification and if something requires additional consideration, we’ll bring it to your attention.
We will construct prototypes and generate test data, and with these outputs and learnings we help take you forward to your next milestone.
Inspect, test and challenge the performance of the concept prototype against the targeted requirements defined in the specification phase.
- Review
Collate the data and learnings and review together with the customer. We help define the options for improvement in the catheter design and provide inputs into future concept specification and prototype iterations and options.
Either the review will feed into the next concept design iteration or we will have enough data and confidence to confirm our ‘Design Select’ and to move to the next phase of your product development.
Development
The Development phase is where your product is evolved from the design select into a commercial product.
- Design Realisation
Your catheter concept is fine-tuned, improved, and optimised with a view to achieving a Design Freeze specification. In design realisation we can consider all elements in the design lifecycle if you require, including handle design, accessory devices, packaging design, sterilisation requirements, manufacturing methods etc.
We look at the total solution for your product in the context of the overall system, considering DFx (design for x) and very specifically DFM (design for manufacture).
- Design Verification and Validation
We take your Design Frozen Specification and assist you in taking it through its Design Verification and Validation process. We work proactively in aiding you to meet your milestone, be that product CE marking, FDA approval or approval for a clinical study.
We support your builds on a developed pilot line with the control, training and traceability you need for your product release.
The final phase of Development is where we make your catheter device manufacturable and scale ready. We manufacture low volume builds during DV, clinical trials and early stages production, and we expand and scale with you as your product enjoys success in the field. Manufacturing may be the final step, but at ICS, manufacturing readiness starts at prototyping and design selection where decisions are made with manufacturing in mind.
Our manufacturing lines process braid and coil assemblies, complex sub-assemblies, and full devices including packaged and labelled devices in volume from our cleanroom facility.
If you have a project coming up and you’d like to talk to one of our engineers about getting an expert insight, give us a call or send an email today. We would love to hear from you.