Challenge us!
Explore the ICS Brainstorming Process
If you have an idea, concept, technical challenge, device/procedural pain points or want to strengthen and expand your device portfolio, the ICS Medical Devices brainstorming process can help you to overcome the challenges and take advantage of the opportunities that exist.
Take a look at our process below and see how the ICS Medical Devices engineering team takes you through the journey, working with you every step of the way.
The building blocks in the ICS Brainstorming Process
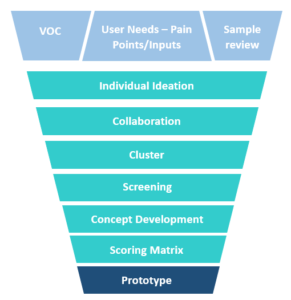
- Ideation
- Collaboration & Cluster
- Screening
It all starts with your input. This can be in the form of drawings, sketches, mock-up products, user needs or pain points, documented or indeed just spoken word.
At ICS, we capture these inputs and requirements and set the targets and boundaries for the brainstorming process; identifying the ‘must haves’, ‘should haves’, ‘could haves’ and ‘must not haves’. The customer will be engaged in this process, supporting information transfer, participating in Q&A, and validating the targets and boundaries that are set for the brainstorming process.
Ideation
Team members on both the customer and the ICS Medical Devices side will independently generate ideas and document them with sketches and descriptions in a fast-paced, dynamic ideation session.
Collaboration & Cluster
Team members come together in pairs or small clusters to review, challenge, add detail and build on the ideas created.
These developing ideas are brought to the wider group for stand-up presentation, further review, challenge, critique, and on-going development. Common ideas are clustered and grouped, ideas are further iterated – the creativity and solution engineering does not stop!
Screening
This step involves applying our collective engineering experience and know-how to select and deselect ideas against the targets and boundaries defined at the start of the process. We often use tools such as the SCAMPER process to aid in screening and further developing the ideas.
Substitute, Combine, Adapt, Modify, Put to another use, Eliminate, Reverse.
The ICS process can take place in a single day but always allow for preparation time, definition of targets and boundaries and information transfer ahead of the brainstorming day.
The output of the ICS brainstorming process should be a developed idea, and hopefully a suite of ideas, that align with our defined targets and boundaries.
____________________
Next Steps
Now that we have our initially developed idea or ideas, ICS Medical Devices takes these ideas through a development process where they are translated to strong developed concepts. The idea can be developed, engineered, and presented in the form of sketches, 3D models, materials specifications, and mock-up where feasible. This development step allows further refinement and evolution of the original ideas.
The brainstorming team, including the customer, connect to review these concepts and to objectively rate, score and measure each one against predefined targets and criteria (many established in the user requirement definition and others added to allow full 360-degree scoring and analysis of the concept). Techniques like applying higher weighting to ‘must haves’ than ‘should haves’ can be useful. This formulaic process removes subjectivity and personal opinion, ensuring the process is objective and true. The scoring is facilitated by ICS Medical Devices but is centred on your review and input, as well as your expertise in the product and clinical requirements for the device.
Concept Development
The development of concepts typically takes 2-5 days, while the review and selection process is shared with the you and can take half a day to a number of days, depending on the time you need to fully digest the concepts and reach consensus on the scoring.
Selected concept or concepts can then be advanced to design, prototype, and testing development cycles. At ICS Medical Devices our objective is to help progress your idea to concept, to prototype, and eventually to a successful product.
The graphic below outlines the process from initial customer input through to prototyping.
To get our engineers involved in your project, contact us today.
Want to know more? Here is a case study where you can see our brainstorming process in action, helping a customer to design a delivery system to deploy a stimulating electrode while minimising tissue damage.